26 Mar A success story: Perspectum Diagnostics Ltd
For the Italian Health System, this is a magical moment. Finally, there is a renewed and genuine attention to addressing the structural health problems of the groups that in Italy are involved in Healthcare Technology Assessment (HTA). In this context, it is essential never to lose sight of the opportunities offered by technological innovation and new emerging clinical practices. Current technology accreditation mechanisms risk ignoring further opportunities.
The history of Perspectum Diagnostic Ltd, hereafter PD, a spin-out (off) of the University of Oxford (UK) is an example. A story of value to tell without any other purpose, an example for the community of health policy makers (radiologists, clinical engineers) and for all young people who gravitate in the STEM area before and after graduation (doctors, biomedical engineers, engineers, physicists, mathematicians).
- Why does talking about this success story represent a virtuous model?
- Why does the business model of PD deserve to be reported to the attention of those who every day decide the direction of our health system?
- Above all, this story may be a valuable example for students and graduates. Students that ought to choose the most suitable university address, or the direction in which to move once graduated to go on the market.
The history of PD – The first scientific evidence dates back to 2007. Doctors, physicists and engineers at the University of Oxford, identified an innovative non-invasive method for the predictive diagnosis and quantification of problems related to diseases of the liver. They propose a technique called LiverMultiScan (LMS) based on the acquisition of MR images of the liver; a biomarker obtained correcting T1 (cT1) sequences. LiverMultiScan brings cT1, T2* and fat fraction together for the first time to support the assessment of chronic liver disease. Unlike other diagnostic approaches that offer quantitative results (US, VCE, CAP, PSWE, SWE, MRE) the LMS achieves three essential goals:
- prevention through validated prognostic indicators;
- classification (staging) of accumulations of iron, fat, fibrosis and inflammation and their quantification without using invasive biopsies (in vivo) that require long response times, special equipment, dedicated staff, risks for patients;
- sensible reduction of management costs to produce validated data for Clinicians useful to make the diagnosis.
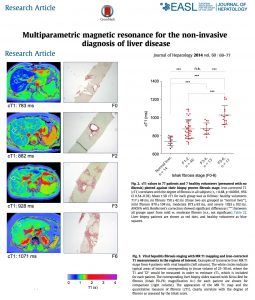
Utility and cost evaluation of multiparametric magnetic resonance imaging – Multiparametric MRI accurately identifies patients with steatosis, stratifies those with NASH (Non-Alcoholic Steatohepatitis) or simple steatosis, and reliably excludes clinically significant liver disease with superior negative predictive value to liver stiffness and ELF (Enhanced Liver Fibrosis). For the risk stratification of NAFLD (Non-Alcoholic Fatty Liver Disease), multiparametric MRI was cost effective and, combined with transient elastography, had the lowest cost per correct diagnosis.
In 2012, the Oxford Centre for Innovation (OCFI) supported the pioneers of Oxford University (Dr Rajarshi Banerjee, Founder & Chief Executive; Sir Michael Brady, Founder, Chairman & Image Analysis Team Lead; Professor Stefan Neubauer, Founder and Chief Medical Officer) for going to the market with a certifiable product. By participating in an EU research project, they eventually kicked off the current Perspectum Diagnostics Ltd (PD). PD began its development and growth, while the Intellectual Property (IP) of the algorithms remained at the University of Oxford.
They worked with the pharmaceutical industry and research centres to gain the CE mark for EU and the 510(k) FDA clearance for the US market (Nov. 2017). In these days, they have about 100 installations; 70% in the US market and the rest between Europe and Australia.
The choice of the logo represents the work program for the future that awaits them: a rainbow of colours that recalls the unique ability to distinguish many different biologic tissues above the scanner shape of an MRI scan.

As many other innovative spin-outs, Perspectum Diagnostics Ltd has defined its own Go-To-Market Strategy (GTM Strategy). On the global market, they present themselves as a trustworthy company certified: ISO9001 Quality Management, ISO / IEC27001 Information Security Management, ISO13485 Medical Device Quality Management, allowing them to work with the pharmaceutical industries, research centres and clinicians with the right cards.
Pharmaceutical Industry: Screen out unsuitable subjects using LiverMultiScan Discover. Reduce biopsy failure rate and access early efficacy signals using our biomarker, corrected T1 (cT1)
Clinicians: We characterise patient’s liver tissue using our FDA-cleared technique and proprietary biomarker, cT1. It sees heterogeneous liver disease non-invasively, aids the clinician to take diagnostic decisions.
Imaging Centers: Access new patient referrals in liver disease and engage in pharma-sponsored research – stay ahead of the curve and grow their business with PD.
Working Model
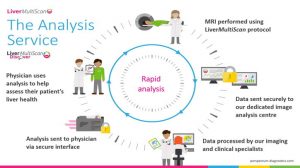
The aces Perspectum Diagnostics Ltd can play are:
- The LMS technology is proposed as a turnkey service for centres that have the most modern equipment or willing to adapt. Customers pay by subscription or consumption.
- Highly competitive system costs [1].
- A non-invasive approach, useful to facilitate the consent of ethics committees.
- The joker, what makes them unique in the global healthcare scene is the possibility of providing prognostic indicators of the evolutions of the pathology that they have started to target. : NAFLD, NASH, inflammation of the liver and other related districts such as gallbladder, pancreas and kidneys.
In short, an idea born in a clinical and bioengineering environment has become a work opportunity for many enterprising young people from all over the world [2], but not only. Spin-outs nurture talents and provide opportunities to learn and grow in an ambitious and dynamic environment. Also, undergraduates who are looking for a placement where their ideas will make a difference are welcome.

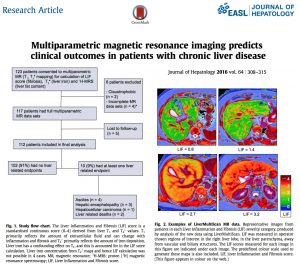
Non-invasive standardised multiparametric MR technology may be used to predict clinical outcomes in patients with chronic liver disease. Perspectum Diagnostics Ltd has also proven to be an opportunity within the health system to treat the patients by doing primary prevention, on the one hand, limiting unnecessary costs, on the other.
Alessandro Mazzarisi, temporary secondee at Perspectum Diagnostics Ltd Oxford (UK) – Marie Curie (RISE)1[1]
Perspectum Diagnostics Ltd https://doi.org/10.1148/radiol.14131890
Disclaimer This is the author’s viewpoint, who is not an employee of PD. The article is not endorsed by Perspectum
[1] Marie Skłodowska-Curie staff members in Research and Innovation Staff Exchanges (RISE) https://goo.gl/JJa3xi
LiverMultiScan Receives FDA 510(k) Clearance for Version 2 of Medical Device
Pharmaceutical Industry – Clinical Trials: LiverMultiScan Discover measures response to treatment.
LiverMultiScan Discover is suitable for monitoring effects during interventional trials.
References:
[1] LiverMultiScan can save £150 per patient – Perspectum Diagnostics https://goo.gl/rAEyHc
[2] Careers https://goo.gl/LgRST2
[3] Marie Skłodowska-Curie staff members in Research and Innovation Staff Exchanges (RISE) https://goo.gl/JJa3xi
[4] Perspectum Diagnostics Ltd is a hepatobiliary medicine service company that provides of the liver and bile ducts https://perspectum-diagnostics.com



No Comments